A recent clinical trial tested a new approach to malaria prevention involving monoclonal antibodies.
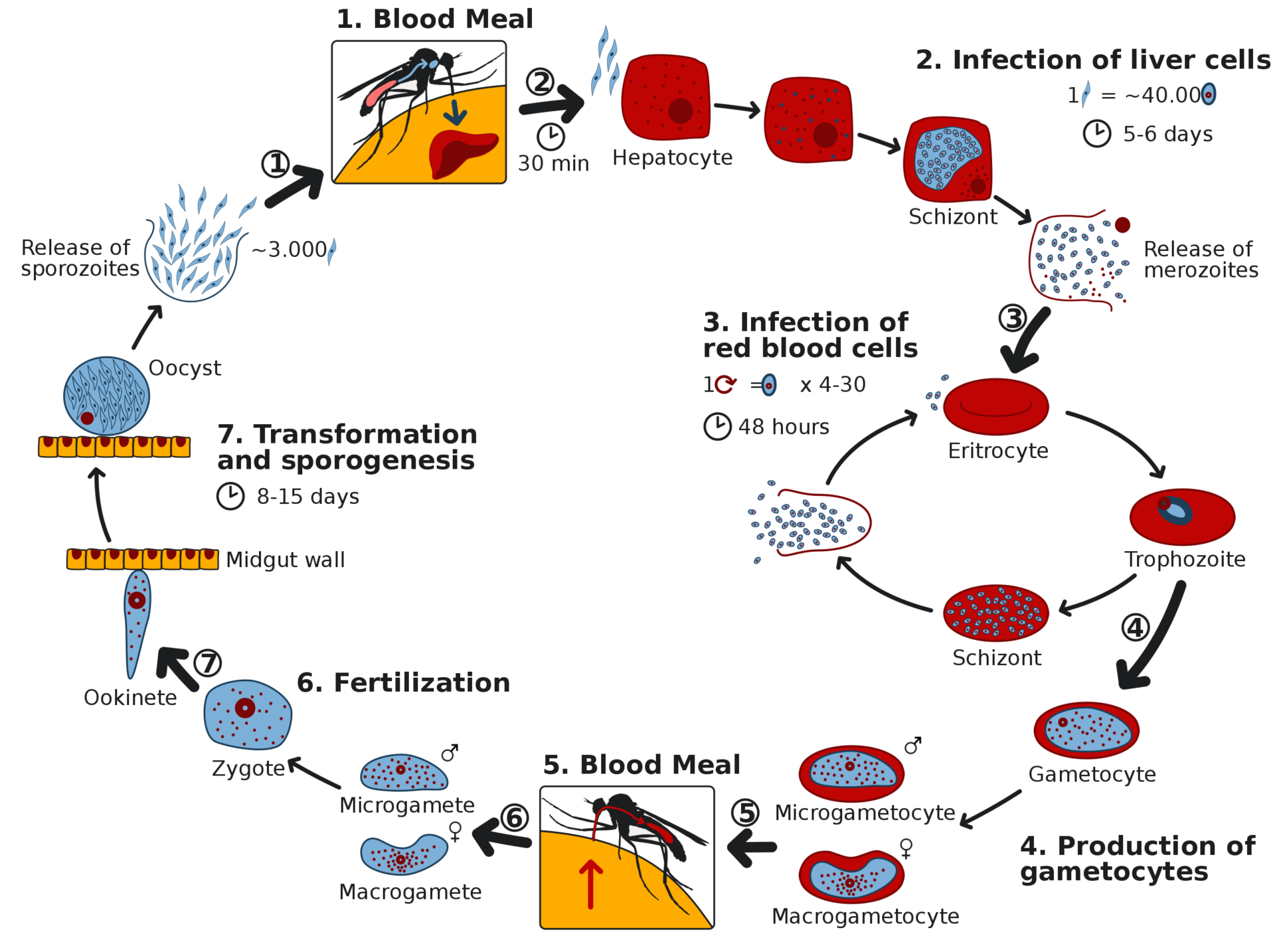
Malaria is a parasitic disease transmitted through mosquito bites. There are 200-400 million new cases each year, with 400,000 annual deaths, plus great morbidity and economic burdens. It has been especially devastating for children in sub-Saharan Africa. Mosquito-control measures, insect repellents, and mosquito nets have been only partially effective. Global infection rates decreased by 50-75% from 2000 to 2015, but the number of cases has continued to rise in many areas despite these interventions. Several drugs are available to prevent and treat malaria, from chloroquine to artemisin, for which the Chinese researcher Tu Youyou was awarded a Nobel Prize in 2015. Unfortunately, drug resistance has diminished their effectiveness. Several vaccines are available or are under development, but they are far from as effective as we might wish.
A phase 1 trial for a new approach
Rather than treating malaria, it would be far better to prevent it in the first place. An article recently published in The New England Journal of Medicine by Gaudinski et al. reported a phase 1 clinical trial of a drug intended to do just that. It was small two-part open-label trial of CIS43LS, a long-acting monoclonal antibody for malaria prevention. In Part A, the drug was given to 25 healthy adults who had not previously had malaria or received a vaccine for malaria; 4 of the 25 received a second dose, and the trial assessed the safety and pharmacokinetics of the drug at one of three escalating dose levels given intravenously or subcutaneously. Seven participants from Part A went on to participate in Part B, in which 11 additional participants were enrolled. 8 people enrolled in Part A and 7 in Part B were left untreated to serve as controls. Finally, a group of participants underwent controlled human malarial infection (after giving informed consent, they were bitten on the forearm by infected Anopheles stephensi mosquitoes); and those who developed parasitemia were treated with atovaquone and proguanil. The protocol was approved by an Institutional Review Board (IRB).
Safety: No safety concerns were identified. All reported adverse events were mild and resolved completely.
Efficacy: A total of 15 subjects were bitten by infected mosquitoes. None of the nine who had received CIS43LS developed parasitemia, while five out of the six untreated controls did, which was consistent with historical data.
Their conclusion: “…malaria can be prevented from 4 to 36 weeks after a single administration of a monoclonal antibody against the major protein covering the surface of the infecting sporozoite.”
Was this statement justified?
Not really. I have several reservations. It was only a small Phase 1 preliminary trial. It was open-label (patients knew when they got the antibody injection). It has not been replicated. They were not able to evaluate subcutaneous injection because of Covid-19-related interruptions in sample collection. The half-life of the monoclonal antibody is only 56 days, so the applicability would be limited to short-term travelers and others for whom a single infusion would eliminate non-compliance and would obviate the need for daily chemoprophylaxis. They studied healthy adults; what is really needed is a way to prevent malaria in children and in the general population. The authors called for further studies, which clearly are needed – at the bare minimum, a good Phase 3 controlled trial will be essential before it could be marketed.
Conclusion: An interesting new approach
These results are intriguing, but monoclonal antibodies will not be acceptable for prevention of malaria until we know more. This is more of a “proof-of concept” experiment. As research continues, I hope more practical antibody treatments will result. We can’t celebrate just yet, but we should stay tuned. That said, it offers a glimmer of hope for malaria sufferers.
This article was originally published in the Science-Based Medicine Blog.
