I could have chosen a prescription eye drop for my dry eyes. I decided not to. Here’s why.
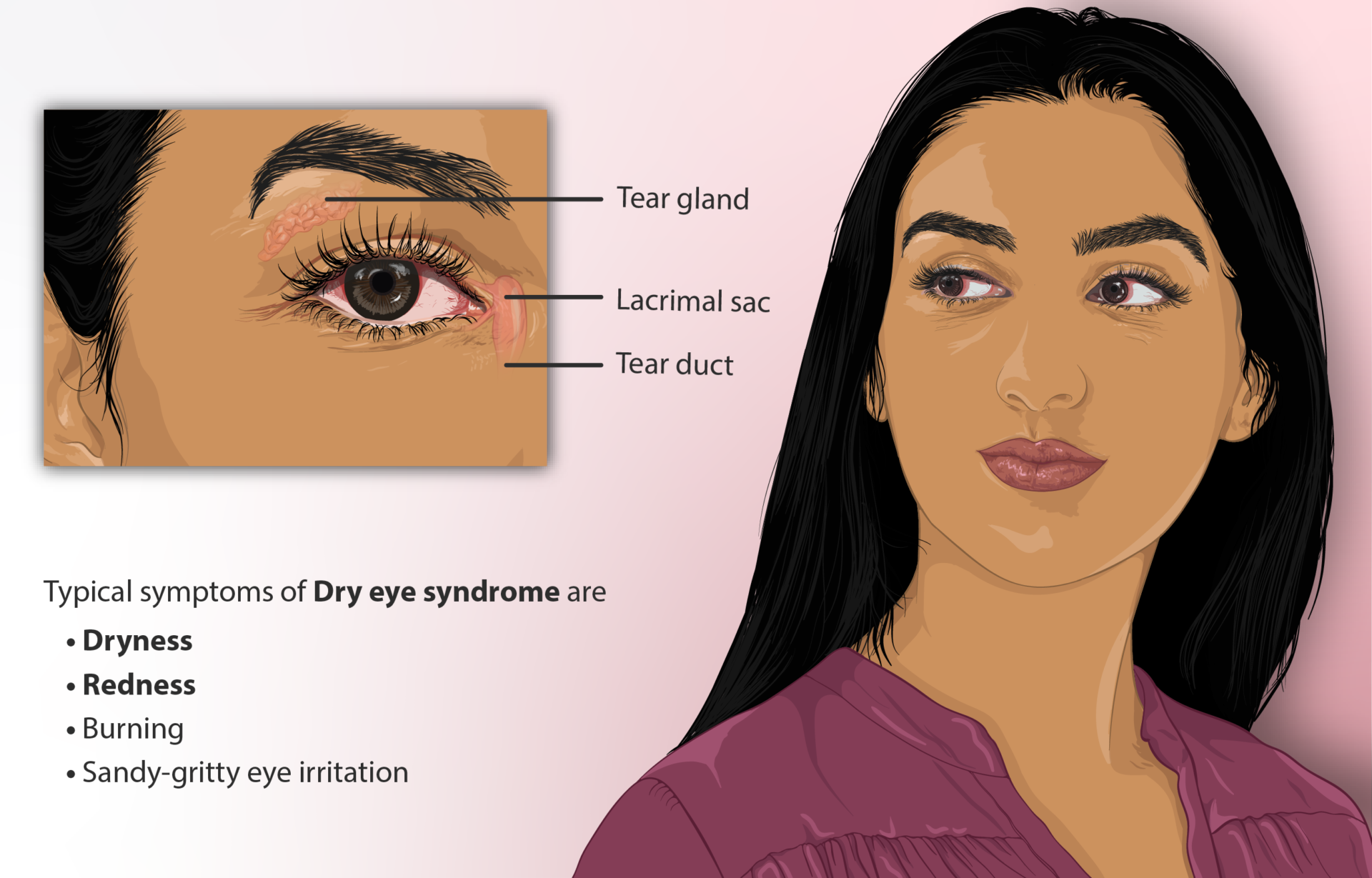
Dry eyes can have many causes: disease, medications, environmental irritants, or just age. Tear production tends to decrease as we get older. My husband (age 80) and I (age 75) were both diagnosed with dry eyes by an ophthalmologist and he instructed us to use lubricant eye drops as often as every hour if needed to maintain comfort. This helps, but it’s cumbersome to use the drops every hour. Well-meaning friends advised me to try prescription cyclosporine eye drops (Restasis and others). After looking at the evidence, I decided not to. Here’s why.
A Cochrane Review of topical cyclosporine in 2019 concluded:
Despite the widespread use of topical [cyclosporine A (CsA)] to treat dry eye, we found that evidence on the effect of CsA on ocular discomfort and ocular surface and tear film parameters such as corneal fluorescein staining, Schirmer’s test, and TBUT is inconsistent and sometimes may not be different from vehicle or artificial tears for the time periods reported in the trials. There may be an increase in non-serious, treatment-related adverse effects (particularly burning) in the CsA group. Topical CsA may increase the number of conjunctival goblet cells. However, current evidence does not support that improvements in conjunctival mucus production (through increased conjunctival goblet cells) translate to improved symptoms or ocular surface and tear film parameters. All published trials were short term and did not assess whether CsA has longer-term disease-modifying effects. Well-planned, long-term, large clinical trials are needed to better assess CsA on long-term dry eye-modifying effects. A core outcome set, which ideally includes both biomarkers and patient-reported outcomes in the field of dry eye, is needed.
This is far from a strong endorsement. They noted that “Artificial tears were used as adjunctive to study medication in all but five trials”.
The Medical Letter reviewed cyclosporine eye drops in 2003 shortly after Restasis was approved by the FDA. The rationale? Dry eye disease is thought to be caused by cytokine-mediated inflammation of the ocular surface with infiltration of T-cells into the lacrimal glands, and cyclosporine has anti-inflammatory and immunomodulating effects. FDA approval was based on two identical randomized, double blind trials involving 877 patients. Cyclosporine was found to be slightly more effective than the vehicle alone in increasing tear production, relieving blurred vision, and decreasing use of artificial tears. Only “slightly” more effective. Transient burning and stinging were reported, but there were no systemic effects. The dose is one drop in each eye twice daily, and the cost is estimated to be $6 a day, or $180 a month. The Medical Letter concluded, “Ophthalmic cyclosporine (Restasis) may improve symptoms and increase tear production in some patients with dry eye disease, but more convincing data are needed. Since blood levels are usually undetectable, this ophthalmic preparation should be safe, but it is expensive.”
Fish oil is endorsed by the American Academy of Ophthalmology, but the evidence is scanty. A randomized double-blind study of fish oil found that the fish oil group had no better outcomes than those who got placebo.
Conclusion: I decided to pass
Considering the cost and the less-than-ringing endorsements, I decided to stick to my lubricant eye drops.
This article was originally published in the Science-Based Medicine Blog.
